MEDICAL CONDITIONING
Context and objectives
The objective of the packaging is to protect the product, to ensure a sterile barrier, to ensure good ergonomics for the end user.


Materials used and supplier
The blister, the tray, the setting is generally done by thermoforming process for example at BLISPAC and generally uses PETG or PET (APET) in the form of rolls. For PETG, the resin manufactured by the chemist EASTMAN is used by extrusion process for example at CAROLEX VITASHEET GROUP.

Device protection
The device must be stalled in a blister to avoid X-Y-Z movements. The robustness of the blister can depend on the thickness of the film used: we can go up to 1.5 mm of thickness of film knowing that on the blister this thickness can be reduced by the stretching which is made to obtain the form. The deeper the room and the steeper sides, the lower the thickness in these areas.
In some cases the blister will be put in another blister, primary conditioning: in contact with the device, secondary-external conditioning to receive the first blister. Validation of the robustness of the packaging is ensured by the transport tests.
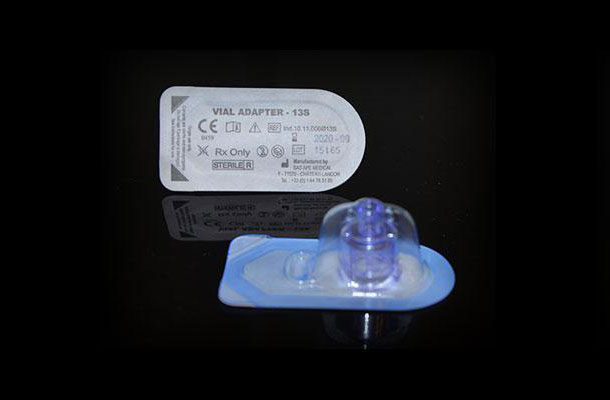
Sterile barrier
Sterilization occurs after conditioning. Therefore, the device must be conditioned and closed in a bacteria-tight manner, that is to say that the blister is thermally sealed with a lid before undergoing radiation or exposure to ETO gas (Ethylene Oxide). Sterilization is provided by suppliers such as IONISOS, STERYLENE, STERIS, STERIGENICS.

To guarantee the tightness over time of the delivered product and thus the sterility, it is necessary to fulfill certain conditions which must be demonstrated at the time of the audits.
For sterilization to be effective, the dose, the exposure of the pallet, or the carton containing the devices must be largely sufficient to kill all the bacteria. It is the sterilization validation process that validates these parameters.
But this validation depends on the quality and nature of the bacteria, molds, and other living organisms present with the device and the package before it is sealed. In order to guarantee a low and controlled level of this level of bacteria, the complete chain of the manufacturing process must be done in clean rooms – white, that is to say in a filtered atmosphere, cleaned regularly and staff equipped with suitable protection. and clean.
If this is not the case, the products must be cleaned and disinfected before being conditioned.
Then the product is put in a blister, then a cover of material like TYVEK of Dupontdenemours or in Medical Paper made by AMCOR or Oliver TOLAS is sealed according to a standard ISO 11607 describing the requirements of keeping the seal.

Do you need additional information?
BLISPAC
Rue de la Gare, 60250 Balagny Sur Thérain
Phone : 03 44 26 25 28
FOLLOW US
CERTIFICATIONS

